Pharmaceuticals in Canada: Industry, Impacts, and Challenges
*1. Background
1.1. What is the industry?
The pharmaceutical industry is composed of companies developing and manufacturing innovative medicines, generic pharmaceuticals, and OTC drug products (Innovation Government of Canada 2011).
1.2. Where is the industry located?
Toronto hosts over 1400 life sciences establishements, including major pharmaceutical companies and research institutions. This makes it a key place for research and commercialization (Toronto Global 2023) .

1.3. Why Toronto?
Concentration of global giants like Amgen, GSK, Roche, Teva, … and proximity to the UofT faculty of medicine and many hospitals that support research. Apotex headquarters are in Toronto (Apotex n.d.).

1.4. What products or services are created?
- Prescription drugs
- Vaccines
- Clinical research services
- Generic drugs(medications created to be the same as an already marketed brand-name drug in dosage, safety, strength, quality, performance, and intended use, but generally at a much lower cost)
- Major local companies like Apotex and Teva Canada produce generic pharmaceuticals for global markets.
1.5. Trade partners
Canada's pharmaceutical sector exports globally, but the united states is canadas main trading partner, with 76.8% of exports and 31% of imports (Innovation Government of Canada 2011).
1.6. Industry level and market process
Pharmaceutical products are heavily regulated. They must go through a multi-stage process that includes RD, clinical trials, regulatory approval, manufacturing, and finally retail. A drug must be tested for safety before being approved by health canada after discovery. If approved, it is manufactured in facilities that follow strict Good Manufacturing Practices, then distributed through wholesaler to pharmacies and hospitals, where patients can access them over the counter or by prescription. This is a tertiary industry.

1.7. Industry history
The pharmaceutical industry in canada began in the early 1900s when companies started producing standardized medicines instead of traditional remedies. Insulin was discovered at the University of Toronto in 1921, giving Canada a key spot in global pharmaceutical innovation. The industry expanded over the 20th century with the growth of international companies and domestic manufacturers such as Apotex, which was founded in 1974. (Canadian Medical Association n.d.; “Empire Laboratories” 2025).
2. Economic Impact
2.1. Economic Impact
The financial value of the Canadian pharmaceutical industry is estimated at $70.51 billion CAD as of 2024 (“Canada Pharmaceutical Market Report and Growth Forecasts 2025-2030: Aging Population Fuels Demand in Canada’s Expanding Pharmaceutical Market” 2025) and it brought $18.4 billion CAD to the Canadian economy's GDP in 2022.. The industry reported an operating revenue of $37.8 billion in RD sectors and a $34.2 billion total output(this includes the value of all goods and services the sector delivered) in output (Huda and Maloney 2022).
The RD sector supported over 110,800 full-time equivalent jobs in 2022 (Huda and Maloney 2022) and the manufacturing portion employed around 35,000 people as of 2024 (Innovation Government of Canada 2011). Also, the sector invested up to $3.2 billion in RD and helped support the majority of clinical trials in Canada, which helps fuel innovation.
2.2. Images
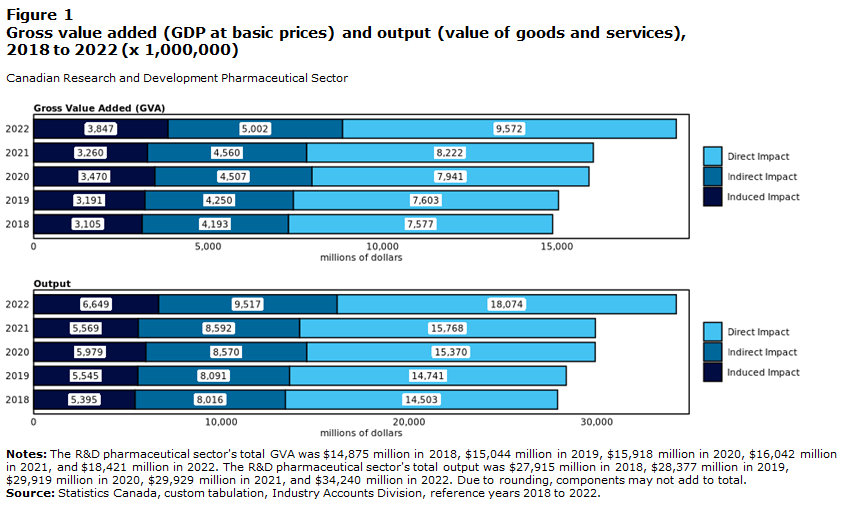
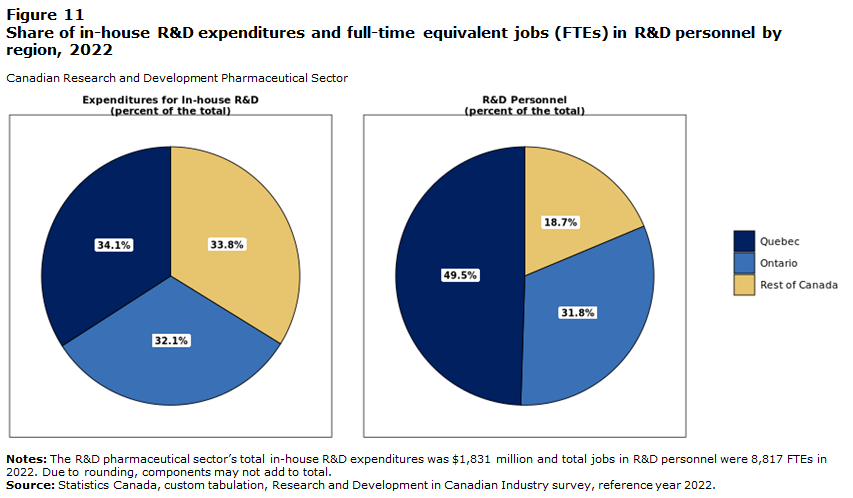
3. Jobs & Careers
3.1. Career paths
- A regulatory affairs specialist manages drug approvals and ensures compliance with Health Canada (“Regulatory Affairs Careers | Pharmaceutical Sciences” n.d.).
- A CRA oversees clinical trials to test new medicines (CCRPS n.d.).
- A pharmaceutical sales representative promotes products to doctors and pharmacists
- You can work manufacturing/quality assurance and make sure drugs are manufactured safely and meet quality standards
- A research scientist does research to discover and improve medications
4. Focus: Regulatory Affairs Specialist
4.1. Daily work
A Regulatory Affairs Specialist ensures pharmaceutical products meet government rules so they can be sold legally.
- Preparing and submitting detailed documentation to regulatory agencies (“Regulatory Affairs Careers | Pharmaceutical Sciences” n.d.)
- Interacting with RD, compliance and manufacturing staff (“Regulatory Affairs Careers | Pharmaceutical Sciences” n.d.).
- Collaborating with scientists, engineers, and legal teams to ensure products follow CGMP (“Regulatory Affairs Careers | Pharmaceutical Sciences” n.d.) .
- Advising companies on regulatory aspects that affect proposed activities (“Regulatory Affairs Careers | Pharmaceutical Sciences” n.d.).
4.2. Challenges
- Regulations change frequently
- High attention to detail is required
- Tight deadlines
- Balancing scientific and business perspectives
4.3. Industry changes
- Increased regulatory complexity (Government of Canada 2025).
- Growth of generic and biosimilars (“Career Paths in the Pharmaceutical Industry | Pharmajob.Ca” 2024)
- Adoption of eCTD submission software
4.4. Wages and job security
Entry-level roles start around ~$76,000 CAD, rising above $142,000 CAD with experience, and senior specialists can earn over $176,000 (“Average Pharmaceutical Regulatory Affairs Specialist Salary in Halifax, Canada for 2025” n.d.). Job security is strong because regulatory compliance is mandatory.
5. Issues & Challenges
5.1. Supply Chain Shortages (Social/Pol)
Canada is experiencing drug shortages due to manufacturing problems, shipping delays, quality disruptions, and shortages of key ingredients (Health Canada 2024).
- Impact
Shortages strain pharmacies and hospitals, increase healthcare costs, and disrupt patient care (Health Canada 2024).
- Significance
Shortages delay treatment and worsen outcomes for vulnerable populations (Health Canada 2024).
5.2. Environmental Sustainability (Environmental/Economic)
The pharmaceutical industry has a large environmental footprint, including greenhouse gas emissions, water usage, chemical waste, and plastic packaging. The industry produces approximately 52 megatons of CO₂ annually (“Understanding the Environmental Impact of the Pharmaceutical Industry | TechTarget” n.d.).
* Impact Environmental concerns increase costs and require investment in green technology (“Sustainability in the Pharmaceutical Industry: Challenges and Opportunities – Golden Aurum Pharma” n.d.).
- Significance
Environmental harm affects ecosystems and public health (“Understanding the Environmental Impact of the Pharmaceutical Industry | TechTarget” n.d.).
6. Potential Solutions
6.1. Supply Chain Shortages
- Implemented solutions
Health Canada has mandatory shortage reporting and mitigation plans. Manufacturers must report shortages and work with partners to reduce impacts (Health Canada 2024, 2022),
- What people can do
- Communicate early with healthcare providers
- Avoid stockpiling medicine
- Report shortages
6.2. Environmental Sustainability
- Implemented solutions
Companies are adopting renewable energy, safer chemicals, recycling, sustainable packaging, and reduced transportation emissions (Finetech 2025).
- What people can do
- Properly dispose of unused medications
- Support environmentally responsible companies
- Reuse and recycle packaging
7. Personal Reflections
7.1. Reflections
In my opinion, the solutions proposed for the pharmaceutical industry’s challenges are mostly effective. Efforts to reduce drug shortages, such as Health Canada’s mandatory shortage reporting and improved supply-chain monitoring, are likely to work because they focus on early detection and coordination between manufacturers, pharmacies, and healthcare providers. However, these solutions might struggle if Canada continues to rely heavily on foreign countries for manufacturing, which makes the supply chain vulnerable to global disruptions. Environmental sustainability solutions are effective but require large investment and public participation. I believe improving supply-chain resilience is the most effective solution because access to essential medicines directly affects patient health and safety. Without reliable access to medications, pharmaceutical practices cannot meet their primary goal of protecting public health.
8. Images
8.1. Maps
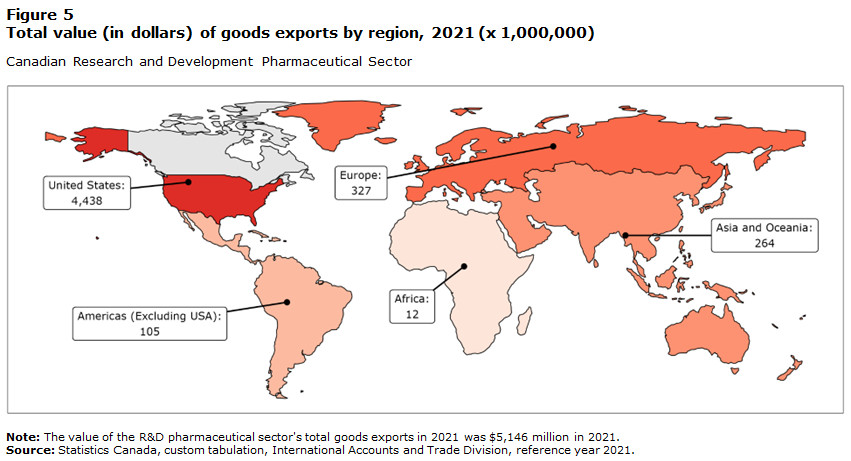
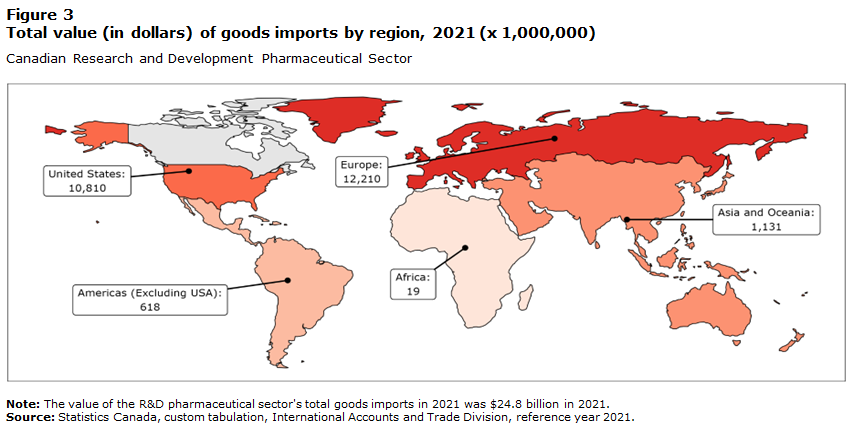
9. Sources
10. Glossaries
10.1. Glossaries and Acronyms
11. Elsewhere
11.1. References
11.2. In my garden
Notes that link to this note (AKA backlinks).
Glossary
Acronyms
